

| Most Common Adverse Events (≥2%) | PLENVU® 2-Day Split-Dosing (n=275) | Suprep® 2-Day Split-Dosing (n=271) |
| Nausea | 7% | 2% |
| Vomiting | 6% | 3% |
| Dehydration* | 4% | 2% |
| Abdominal Pain/Discomfort† | 2% | 2% |
| Decline in Glomerular Filtration Rate (GFR)‡ | 2% | 2% |
| Electrolyte abnormalities§ | 2% | 1% |
| Fatigue | 2% | 1% |
| Headache | 2% | 1% |

However, elderly patients are more likely to have decreased hepatic, renal, or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities.1
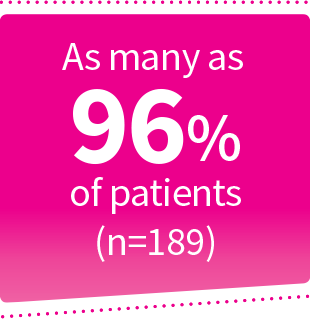
Percentage reflective of patients who participated in the NOCT and MORA trials. Mild renal insufficiency was defined as a creatinine clearance (CrCl) ≥60 mL/min to <90 mL/min. Moderate renal insufficiency was defined as CrCl ≥30 mL/min to <60 mL/min.2
||Successful Cleansing—Grade A and B. For Grade A, there must be full visibility of the entire bowel mucosa before precleaning. For Grade B, all 5 bowel segments must contain only brown liquid/fully removable semisolid stools, clear liquid, or be empty and clean2
References: 1. Plenvu. Prescribing information. Salix Pharmaceuticals, a division of Bausch Health US, LLC; 2023. 2. Data on file. Salix Pharmaceuticals.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please click here for full Prescribing Information.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
PLENVU® is contraindicated in patients with gastrointestinal (GI) obstruction, bowel perforation, gastric retention, ileus, toxic megacolon, and hypersensitivity to any of its ingredients.