

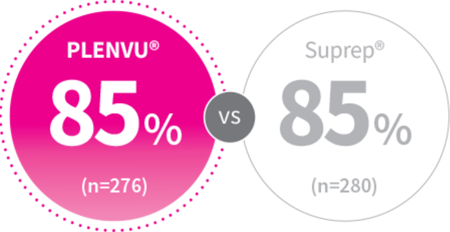
mITT: P=.528
2-Day Split-Dosing
Patients, %
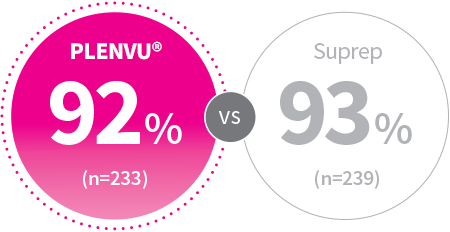
PP: P=.600
2-Day Split-Dosing
Patients, %
NOCT Trial Study Design: Randomized, multicenter, colonoscopist/central reader-blinded phase 3 noninferiority study assessed the bowel-cleansing efficacy, safety, and tolerability of PLENVU® vs Suprep in 556 adults on a 2-day split-dosing regimen using the validated Harefield Cleansing Scale (HCS).1,2
The HCS criteria are applied after precleaning of the colon3
(NOCT Trial) Colonoscopist/Central Reader*
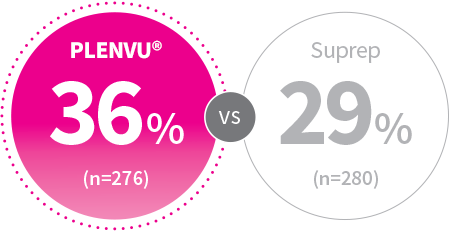
mITT: P=.059
2-Day Split-Dosing
Patients, %
(Post Hoc) On-Site Colonoscopist†
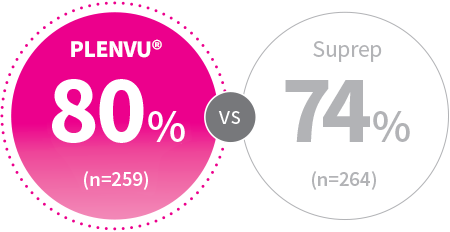
mITT: P=.079
2-Day Split-Dosing
Patients, %
*Trained central readers assessed the efficacy of bowel cleansing based on video review, using both the HCS and the Boston Bowel Preparation Scale (BBPS)2
†This post hoc analysis shows the cleansing assessment by the site colonoscopists, who typically guide clinical decision-making; hence this analysis may be useful for clinical practice. In the NOCT study, 621 patients (males and females, aged 18–85 years) were randomly assigned in a 1:1 ratio to receive either PLENVU® or Suprep, each administered as an overnight split-dose. Data from the 523 patients who underwent a colonoscopy and had a site colonoscopist assessment were used in this analysis. Colonoscopists were blinded to the preparation administered. Cleansing was assessed according to the HCS; segmental scores 3 and 4 were judged as high-quality cleansing4
mITT, modified intent-to-treat: Used as the primary population for all efficacy analyses and included all randomized patients except for any patient who was randomized but subsequently failed to meet entry criteria or if the same patient did not receive any study drug2
PP, per protocol: Included patients without major protocol deviations, who met eligibility criteria, who took at least 75% of each bowel prep, and who had available data for at least 1 of the primary end points2
References: 1. Plenvu. Prescribing information. Salix Pharmaceuticals, a division of Bausch Health US, LLC; 2023.. 2. DeMicco MP et al. Gastrointest Endosc. 2018;87(3):677-687.e3. doi:10.1016/j.gie.2017.07.047 3. Data on file. Salix Pharmaceuticals. 4. Epstein M et al. Poster presented at: WCOG at ACG 2017; October 13-18, 2017; Orlando, FL. Presentation number P1789.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please click here for full Prescribing Information.
PLENVU® (polyethylene glycol 3350, sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride for oral solution) is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
PLENVU® is contraindicated in patients with gastrointestinal (GI) obstruction, bowel perforation, gastric retention, ileus, toxic megacolon, and hypersensitivity to any of its ingredients.